End-of-wear report with 99% physician agreement1
Expertly curated reports using FDA-cleared, deep-learned AI and verified by Qualified Cardiac Technicians — resulting in 99% agreement from physicians.1-5
Reporting
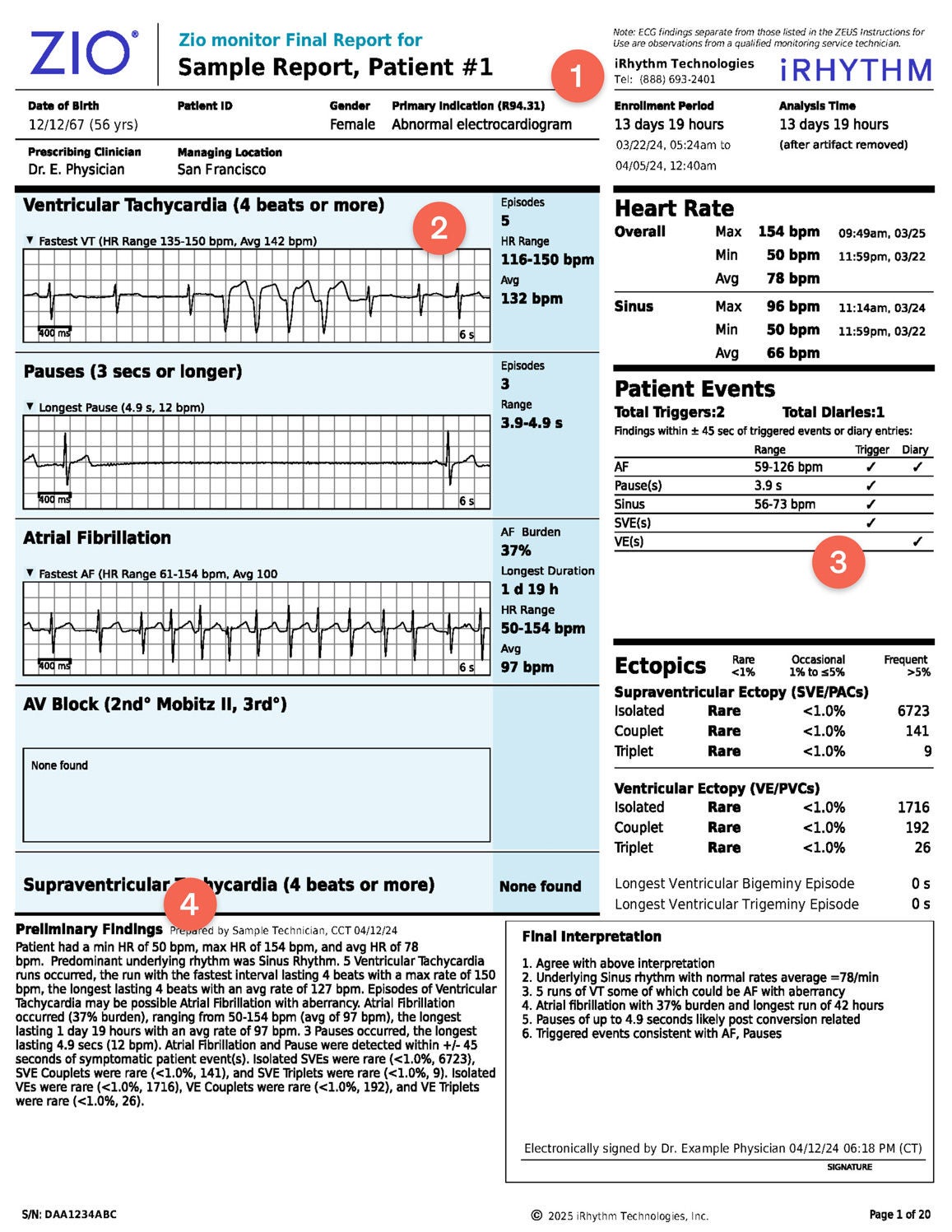
1. The Zio® report cover page provides a snapshot of actionable and clinically significant findings. The upper-right corner of the report details the wear time and the total analyzable time with artifacts removed.
2. Representative ECG strips are arranged in order of clinical significance with additional at-a-glance information such as duration, episodes, and burdens
3. Navigate with one click from the cover page to any of the report sections for more details. Download the report for the full experience.
4. Preliminary findings in the Zio report are intended for use by clinicians as an aid in arrhythmia diagnosis and management.
Get a comprehensive Zio Report
Best-in-class deep learned algorithm
Recorded ECG data is processed for detection of arrhythmias using an FDA-cleared, deep learned algorithm that can detect 13 types of arrhythmias, plus sinus rhythm and artifact. The data is then curated and verified by Qualified Cardiac Technicians (QCTs) to generate a Zio end-of-wear report.2-5

The human side of the equation.
Because of our commitment to accuracy, our team of Qualified Cardiac Technicians reviews and curates 100% of our reports before you receive them. And since human reviewers are part of every report, your patients’ well being is thoughtfully considered.
As elegant and easy to navigate as it is powerful.
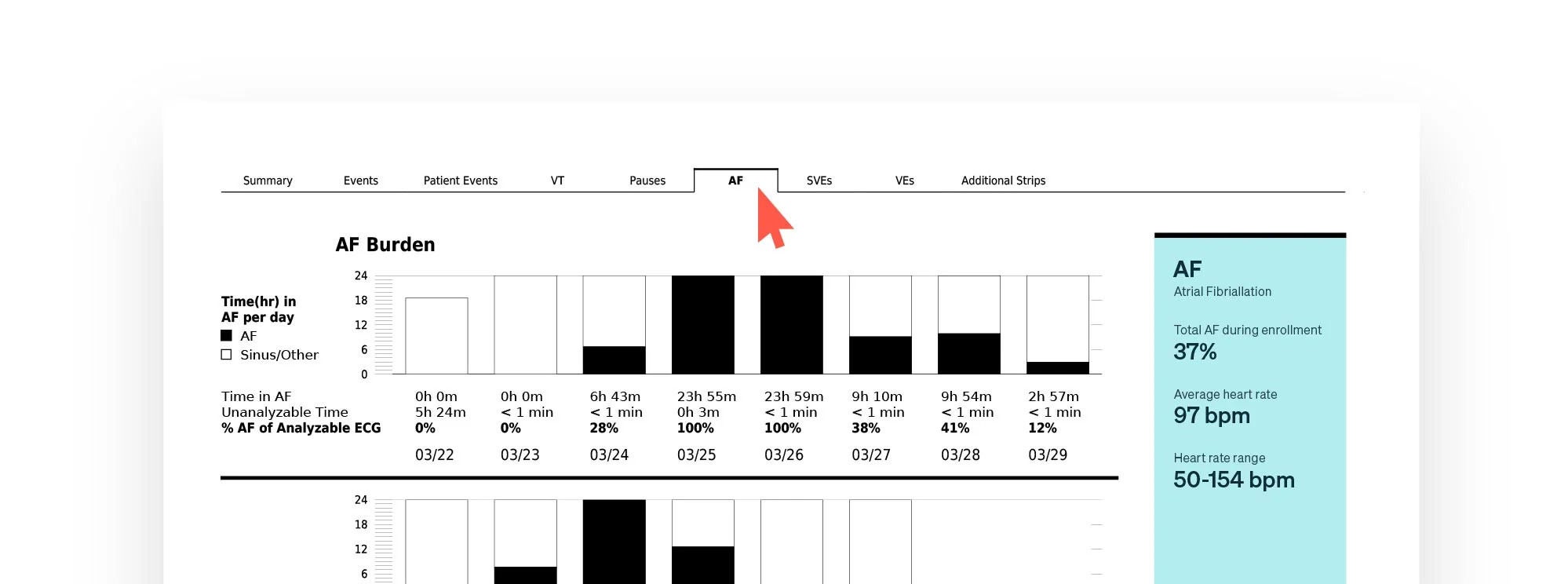
The Zio report provides you with a rich summary of the most important cardiac events right on the first page — and an easy-to-navigate deep dive for all the intricate details captured.
It’s also available at your fingertips on the ZioSuite mobile app.
Continue the conversation

- 99% of physicians agree with the comprehensive end-of-wear report. Based on a review of all online Zio XT, Zio monitor, and Zio AT end-of-wear reports. Data on file. iRhythm Technologies, 2023.
- Data on file. iRhythm Technologies, 2020.
- Hannun et al. Cardiologist-level arrhythmia detection and classification in ambulatory electrocardiograms using a deep neural network. Nat Med. 2019;25:65-69. https://doi.org/10.1038/s41591-018-0268-3
- Deep learned algorithm is only available in the United States, European Union, Switzerland, United Kingdom, and Japan.
- FDA 510K clearance, CE mark, UKCA mark, and PMDA-approval.
- Data on file. iRhythm Technologies, 2025.
- Data on file. iRhythm Technologies, 2024.
WEB0008.03